Enhancing Product Safety with WHO-GMP
Understanding the Importance of WHO-GMP Standards
In the pharmaceutical world, patient safety begins with the quality of every medicine produced. Each tablet, capsule, and injectable carries a commitment to human well-being, and that commitment is safeguarded by compliance with WHO-GMP — the World Health Organization’s Good Manufacturing Practices. These globally recognized standards serve as the foundation for ensuring that medicines are consistently produced, controlled, and tested according to strict quality norms.
WHO-GMP guidelines go beyond basic regulatory requirements; they represent a systematic approach to manufacturing that integrates hygiene, process validation, documentation, and personnel training. Their purpose is simple yet critical — to minimize risks associated with pharmaceutical production and ensure that products are safe, effective, and of high quality.
At its core, WHO-GMP compliance ensures that every step in the manufacturing process — from procurement of raw materials to packaging and storage — is carried out under well-defined, validated, and monitored procedures. This framework prevents contamination, reduces variation, and guarantees batch-to-batch consistency.
For manufacturers, adopting WHO-GMP is not just about meeting regulatory obligations; it’s about earning trust. Patients, healthcare providers, and partners rely on assurance that every dose meets global standards for safety and efficacy. As the pharmaceutical market expands and competition intensifies, companies that strictly follow WHO-GMP guidelines demonstrate a clear commitment to excellence.
Moreover, WHO-GMP certification opens doors to international markets by validating a company’s capability to produce medicines that meet global expectations. It ensures credibility, builds brand reputation, and provides a competitive edge in an industry where quality defines success. For any pharmaceutical company aiming for long-term sustainability, WHO-GMP is not merely a certificate — it is the very essence of responsible manufacturing.

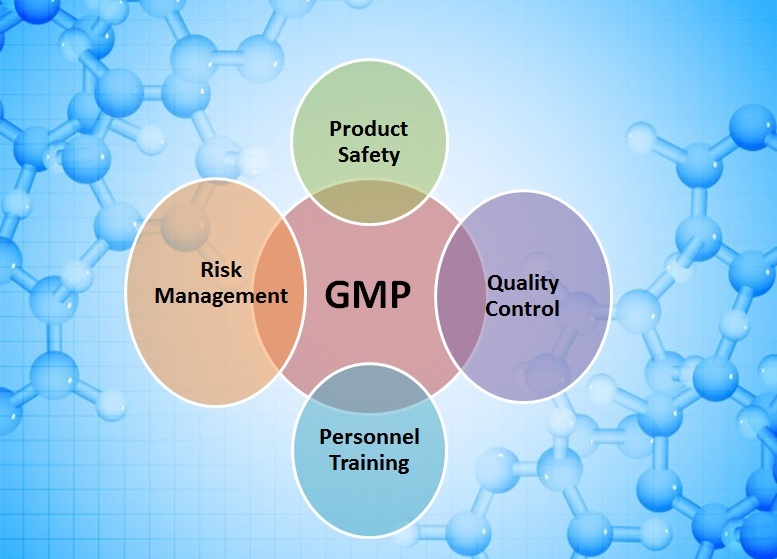
Key Components of WHO-GMP and Their Role in Quality Assurance
WHO-GMP guidelines are built upon several core principles that collectively safeguard the integrity of pharmaceutical production. These principles define how a manufacturing facility should operate, what systems should be in place, and how risks must be managed to ensure product safety and consistency.
- Quality Management System (QMS) : A robust Quality Management System is the foundation of WHO-GMP compliance. It ensures that all operations are planned, executed, and monitored under controlled conditions. This system includes standard operating procedures (SOPs), documentation practices, and defined responsibilities that guarantee traceability and accountability across every process.
- Qualified Personnel : Human resources play a pivotal role in pharmaceutical manufacturing. WHO-GMP mandates that only trained and qualified personnel handle production, testing, and packaging. Continuous training ensures that employees are aware of safety protocols, contamination prevention, and quality standards, thus maintaining operational excellence.
- Premises and Equipment : A clean, well-designed facility is crucial for maintaining product purity. WHO-GMP guidelines specify strict requirements for building layout, air handling, temperature control, and equipment maintenance. This prevents cross-contamination and ensures that the environment supports consistent product quality.
- Raw Material Control : All raw materials used in production must be verified, tested, and approved before use. WHO-GMP requires detailed supplier qualification and sampling procedures to ensure that only the best-quality materials enter the production cycle. Proper storage and handling also prevent degradation or contamination.
- Production and Process Control : Every stage of production — from mixing to filling and packaging — must follow validated procedures. These processes are continuously monitored for compliance, accuracy, and precision. The use of process validation ensures that equipment, parameters, and methods yield consistent results.
- Quality Control and Testing : Comprehensive testing of raw materials, in-process samples, and finished products ensures the highest level of safety and reliability. Analytical methods must be validated, and results must be properly documented. This step confirms that the final product meets the desired specifications for potency, purity, and stability.
- Documentation and Record Keeping : “Write what you do and do what you write” is a fundamental GMP principle. Every action, process, and test must be documented to establish a clear history of each product batch. Proper documentation ensures transparency, accountability, and traceability in case of audits or product recalls.
- Self-Inspection and Continuous Improvement : Regular internal audits help identify gaps and improve processes. WHO-GMP encourages manufacturers to establish a culture of continuous improvement, where feedback, corrective actions, and preventive measures drive long-term excellence.
Each of these components works together to ensure that the manufacturing process is robust, controlled, and aligned with global safety expectations. By implementing WHO-GMP principles, pharmaceutical companies not only protect their consumers but also strengthen their operational efficiency and market reputation.
How WHO-GMP Ensures Product Safety, Consistency, and Global Trust
The true strength of WHO-GMP lies in its ability to transform manufacturing practices into a well-coordinated system of checks, balances, and controls that prevent errors before they occur. Through its structured approach, WHO-GMP ensures that every product reaching the patient is safe, consistent, and therapeutically effective.
- Minimizing Risk through Preventive Measures
WHO-GMP places a strong emphasis on risk assessment and prevention. By identifying potential hazards early — whether related to materials, machinery, or human error — manufacturers can implement control measures to avoid contamination or product failure. Preventive maintenance, calibration, and environmental monitoring all contribute to a safer production environment.
- Ensuring Batch Consistency
Every batch produced must match the established quality profile of the product. WHO-GMP guidelines demand validation of all production processes to eliminate variability. Consistency in mixing, granulation, compression, and packaging ensures that each dose delivers the intended therapeutic effect.
- Contamination Control
Cross-contamination can compromise patient safety and brand integrity. WHO-GMP mandates stringent hygiene and sanitation measures, cleanroom protocols, and controlled material flow to minimize contamination risks. Special zones, filtered air systems, and dedicated equipment are used to maintain purity.
- Product Traceability and Recall System
One of the cornerstones of WHO-GMP is traceability. From raw material procurement to distribution, every step is documented to track the product’s history. In rare cases of product deviation, this allows for swift and targeted recalls, protecting both consumers and company reputation.
- Regulatory Compliance and Market Access
Products manufactured under WHO-GMP standards meet the expectations of global regulatory authorities such as the USFDA, EMA, and TGA. This compliance not only enables exports but also enhances consumer confidence in international markets.
- Enhanced Patient Safety
Ultimately, WHO-GMP compliance translates into safer medicines for patients. By ensuring precision in formulation, dosing, and stability, it minimizes the risk of adverse effects, contamination, or therapeutic failure. Each tablet or capsule becomes a reflection of the manufacturer’s integrity and responsibility.
- Brand Reputation and Trust
In an industry where reputation is everything, WHO-GMP certification acts as a seal of reliability. Companies that operate under these guidelines demonstrate a commitment to quality that resonates with healthcare professionals and consumers alike. It builds long-term trust, encourages repeat business, and strengthens the brand’s global presence.
- Driving Continuous Innovation
By maintaining strict standards, WHO-GMP fosters a culture of innovation and accountability. Manufacturers continually upgrade technology, adopt automation, and refine processes to stay compliant and competitive. This pursuit of excellence leads to safer, more effective, and more advanced pharmaceutical products.
Through these principles, WHO-GMP transforms pharmaceutical manufacturing into a process where safety, quality, and compliance become inseparable. It enables companies to maintain consistency, achieve operational excellence, and build a global reputation based on integrity and trust.
WHO-GMP at the Core of VTV Formulations’ Quality Commitment
At VTV Formulations, WHO-GMP compliance is more than a regulatory requirement — it is the foundation of our manufacturing philosophy. Every product we create, from tablets and capsules to injectables, is backed by a system designed for precision, purity, and reliability.
Our facilities are built and maintained according to WHO-GMP specifications, ensuring optimal environmental control, process validation, and equipment calibration. Every production stage is monitored by trained professionals, and every batch undergoes rigorous testing before approval. This meticulous attention to detail ensures that clients receive products that are safe, consistent, and of unmatched quality.
We integrate WHO-GMP principles into our core operations through:
- Comprehensive Quality Assurance : Every raw material, process, and finished product is verified under strict protocols.
- Advanced Infrastructure : Our facilities feature modern cleanrooms, HVAC systems, and automated equipment that reduce human error.
- Continuous Training : Our skilled workforce undergoes ongoing GMP and safety training to maintain operational excellence.
- Strong Documentation Practices : Each batch record, analytical report, and validation document is securely maintained for traceability.
- Timely Delivery and Efficiency : We combine compliance with efficient production planning to ensure on-time delivery without compromising quality.
By adhering to WHO-GMP guidelines, VTV Formulations ensures that our clients’ products meet international standards and earn customer trust across global markets. Our unwavering focus on safety, quality, and compliance makes us a preferred partner for pharmaceutical companies seeking reliability and excellence.
Conclusion
In today’s rapidly evolving pharmaceutical landscape, WHO-GMP standards are more than a regulatory expectation — they are the backbone of global product safety and trust. By following these stringent guidelines, manufacturers like VTV Formulations not only safeguard patient health but also uphold the integrity of their brand and the industry as a whole.
Our commitment to WHO-GMP reflects our promise to deliver safe, effective, and reliable medicines that meet the highest international standards. Through innovation, transparency, and an unyielding focus on quality, we continue to build a healthier and more confident world — one formulation at a time.
Explore Related Blogs
Stay informed with our curated selection of similar blogs, offering expert perspectives on pharmaceutical trends, regulatory updates, and product innovations.
These articles are designed to help healthcare professionals, partners, and businesses stay ahead in an ever-evolving industry. Explore more to deepen your knowledge and make informed decisions.